
Welcome to Watmind USA – Home of SpeedySwab™ Rapid COVID-19 + Flu A&B Antigen Self-Test
Watmind USA is a leading healthcare biotechnology company committed to providing innovative diagnostic testing devices to U.S. consumers. Our mission is to improve healthcare outcomes by delivering reliable, fast, and accurate testing solutions. At the heart of this mission is our flagship brand, SpeedySwab™, which sets the standard for COVID-19 Flu A + B test innovation. By prioritizing accuracy, speed, and convenience, SpeedySwab™ ensures that individuals and healthcare providers have access to the most efficient at-home COVID flu test and COVID flu self-test options on the market.
With a focus on meeting the needs of individuals, families, and healthcare providers, Watmind USA is proud to offer a range of products, including the widely recognized SpeedySwab™ Rapid COVID-19 + Flu A&B Antigen Self-Test. This COVID flu combo test allows consumers to detect COVID-19, Flu A, and Flu B with one simple nasal swab, providing fast results from the comfort of home. Available for purchase online and at major retailers, this user-friendly, FDA-approved COVID flu test is designed to be accessible, affordable, and easy to use.
Innovation, reliability, and user-centered care are the core values that drive Watmind USA’s approach to diagnostic testing. Our goal is to ensure that every person has access to fast, accurate testing—whether they’re searching for a “COVID flu test near me”, looking for a COVID flu home test, or in need of a same-day COVID flu test. With support for home use and professional healthcare settings and major pharmacies, Watmind USA empowers consumers with testing options that prioritize both health and convenience.
Introducing Speedy Swab™

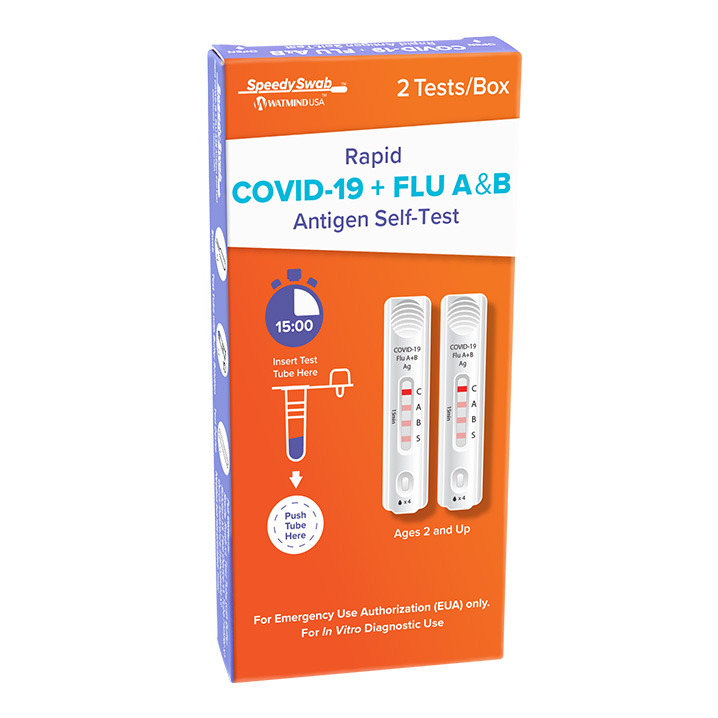
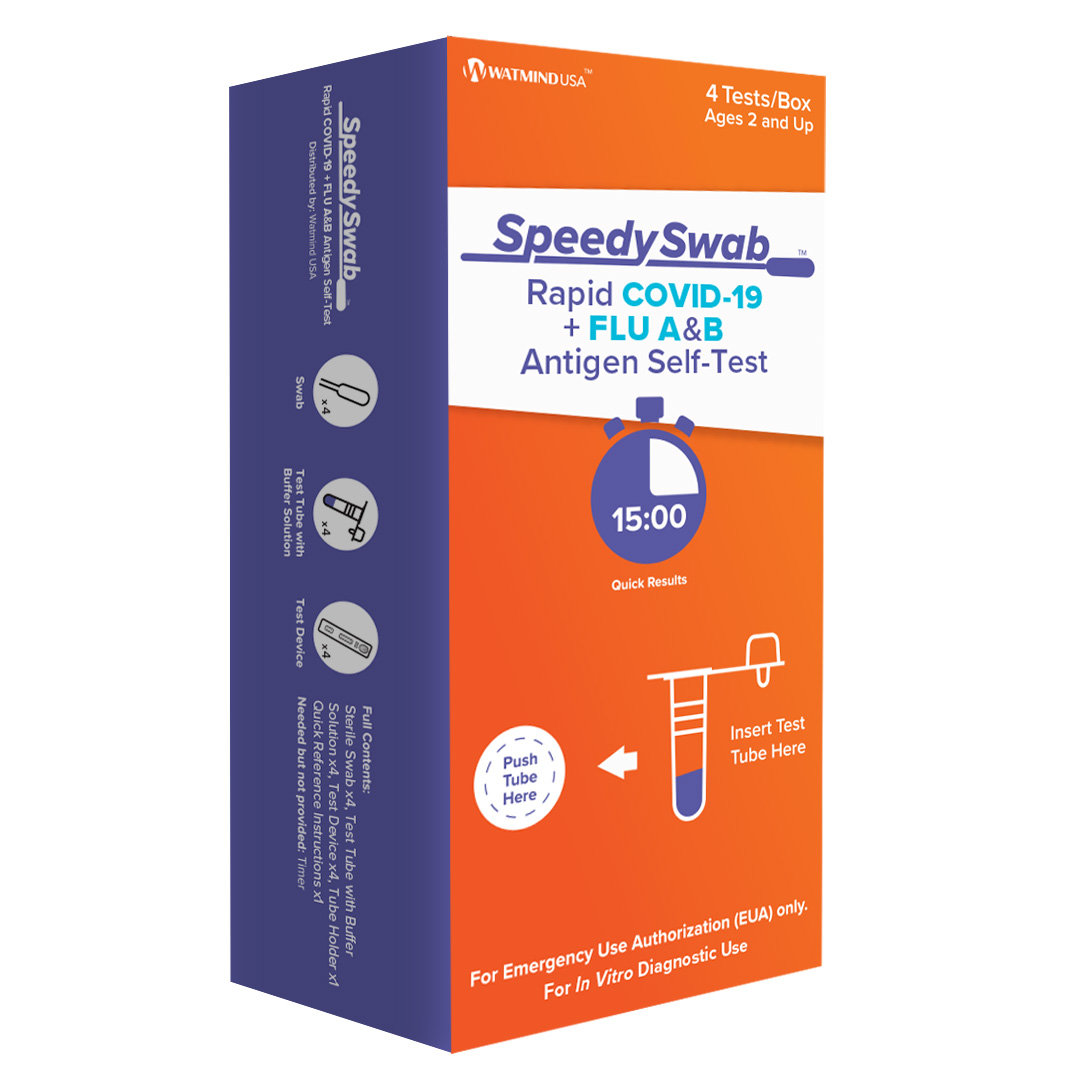
Get fast, accurate results with the SpeedySwab™ Rapid COVID-19 + Flu A&B Antigen Self-Test – the ultimate solution for detecting COVID-19, Flu A, and Flu B in a single, easy-to-use nasal swab test. Designed for both at-home COVID flu testing and use by healthcare providers, this combined COVID flu combo test allows you to test for multiple viruses at once, saving you time and money. Now you can have peace of mind with accurate results delivered in minutes, all from the comfort of home.
The SpeedySwab™ is an FDA Emergency Use Authorized (EUA) COVID flu test authorized for non-prescription home use. It works with self-collected anterior nasal swabs for individuals aged 14 and older or adult-collected nasal swabs for children aged 2 and older. This convenient, COVID flu self-test offers unmatched speed, simplicity, and accuracy, similar to our trusted SpeedySwab™ Rapid COVID-19 Antigen Self-Test. Whether you’re searching for a COVID flu test near me or looking for a COVID flu home test available for same-day delivery, SpeedySwab™ has you covered.
Our affordable and widely accessible test is available online and from leading retailers. With its intuitive design and proven accuracy, it’s no wonder SpeedySwab™ is the top choice for families, travelers, and healthcare providers. Looking for fast results? Order your COVID flu test kit online or pick up in-store or check availability for same-day COVID flu test options at your nearest grocery store or pharmacy. Whether it’s a work requirement, school safety, or personal health check, SpeedySwab™ makes it easy to know your status fast.
Why You Should Test for COVID-19 and Flu?
If you’re feeling sick with symptoms like a sore throat, fever, cough, or body aches, it can be hard to tell if you have COVID-19, the flu, or just a common cold. Since these illnesses have similar symptoms, using a COVID-19 and Flu test kit can give you clear answers fast. The CDC (Centers for Disease Control and Prevention) advises that testing is the only reliable way to know which virus you have. Knowing whether it’s COVID-19, Flu A, or Flu B helps you avoid spreading the illness to family, friends, and coworkers. It also helps you get the right treatment — since flu and COVID-19 have different treatments.
Testing early can make a big difference, especially for young children, older adults, and people with underlying health conditions. According to the CDC, early treatment for flu and COVID-19 is most effective when starting within the first few days of symptoms. Medications like antivirals can shorten illness duration and prevent serious complications, but only if you know what you’re dealing with. An at-home COVID and flu test gives you quick results, so you can act fast and get treatment if needed. Without a test, you could be delaying care that might keep you out of the hospital.
Beyond personal health, COVID-19 and flu testing helps protect your community. When people know if they have COVID-19 or the flu, they can make better choices about staying home, wearing masks, and avoiding close contact with others. The CDC uses test data to track virus outbreaks and identify where the flu and COVID-19 are spreading most. This information helps public health officials make recommendations on vaccines, health alerts, and response plans. By testing, you’re not only protecting yourself — you’re also helping keep your loved ones and community safe.
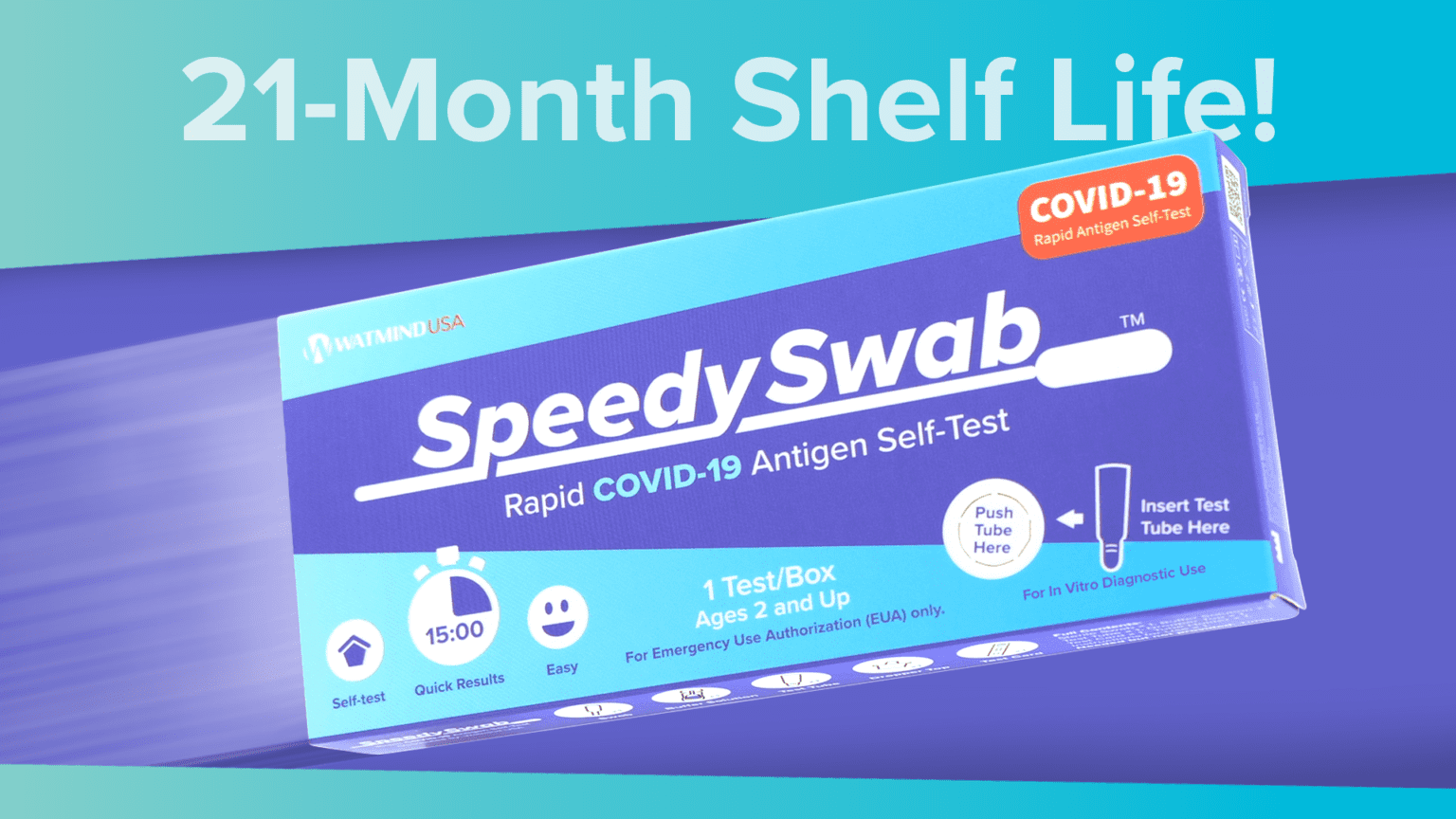
The SpeedySwab™ COVID-19 Antigen Self-Test is an FDA EUA-authorized at-home diagnostic test designed for quick, accurate detection of COVID-19 and Flu. With a 21-month shelf life and a simple nasal swab method, this test ensures reliable identification of SARS-CoV-2 variants and sub-variants, as well as flu viruses if using the SpeedySwab™ COVID-19 Flu Antigen Self-Test (above).
Using the trusted Lateral Flow Assay (LFA) technology, this COVID antigen test provides fast and clear results in just 15 minutes. The process is as simple as:
- Swirl the nasal swab inside each nostril for 15 seconds.
- Place the swab into the test tube containing a buffer solution.
- Apply 3 drops from the tube onto the test card.
The test delivers fast and clear results, giving users the confidence they need at home. This at-home COVID flu test offers the convenience of testing from home without the need for a healthcare provider.
Perfect for those seeking a COVID flu combo test or a COVID rapid antigen test, SpeedySwab™ stands out as a trusted choice for families and individuals. For those wondering about COVID test availability, SpeedySwab™ can be found in major retailers and online.
With its user-friendly design and reliable results, SpeedySwab™ makes COVID self-testing simple, accessible, and efficient for anyone needing to check for both COVID-19 and Flu in one go.
Watmind USA Speedy Swab™ Exclusive Distributor
Biolabs International LLC
7887 Dunbrook Rd Ste H
San Diego, CA 92126
US +1 800-440-4580




